RESEARCH ARTICLE
Acoustic Transmitters Impact Rainbow Trout Growth in a Competitive Environment
Tanner J. Urbaniak1, Michael E. Barnes2, *, Jacob L. Davis1
Article Information
Identifiers and Pagination:
Year: 2016Volume: 9
First Page: 37
Last Page: 44
Publisher Id: TOFISHSJ-9-37
DOI: 10.2174/1874401X01609010037
Article History:
Received Date: 24/02/2016Revision Received Date: 27/04/2016
Acceptance Date: 13/05/2016
Electronic publication date: 10/08/2016
Collection year: 2016
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Fish implanted with acoustic transmitters are assumed to behave and grow after stocking similar to untagged fish. In this study, three groups (tagged, sham, and control) of rainbow trout Oncorhynchus mykiss [mean (SD) initial length = 277 (24) mm] range were maintained together in three raceways for 90 days, with each raceway containing 10 tagged, 10 sham, and 10 control fish. The fish in the tagged group were anesthetized and had an inert transmitter inserted via a ventral incision. Fish in the sham group were anesthetized and had an incision without transmitter implantation, while the control group was anesthetized only. In each raceway, trout with the inert transmitters were significantly lighter and shorter than fish from the other two groups at the end of the experiment. However, the reduction in weight, length, and specific growth rate occurred primarily during the first 38 days post-tagging, with tagged fish growing at similar rates to the other two groups for the final 52 days of the experiment. Mortality data indicated a survival threshold of 280 mm length in the tagged fish, with 100% survival of the Rainbow Trout greater than 280 mm and only 59.1% survival of trout less than 280 mm. Based on the results of this study, rainbow trout implanted with 9 x 24 mm, 3.6 g acoustic transmitters should be held prior to release for a minimum of 38 days to ensure similar growth rates as untagged conspecifics, and only trout with an initial length greater than 280 mm should be used to maximize survival.
INTRODUCTION
Acoustic telemetry is widely used to determine fish movement and survival [1]. Acoustic transmitters are surgically-implanted into the fish, with the assumption that the movement, behavior, and survival of the tagged fish are unaffected [2, 3]. In order to produce valid data, it is essential that acoustic tags and the surgery required to implant them have negligible impacts, so that the behavior and fate of tagged fish is similar to untagged conspecifics [4].
The results from other studies examining the impacts of acoustic tags on implanted fish are inconsistent. Acoustic tag implantation in juvenile Chinook salmon Oncorhynchus tshawytscha has been shown in some studies to have no deleterious effects on predator avoidance or swimming performance, and no negative effects on long-term growth [1, 2, 5]. Similarly, no negative effects of acoustic transmitter implantation on the growth sockeye salmon Oncorhynchus nerka have been observed [6]. Atlantic salmon Salmo salar that were implanted with acoustic transmitters, underwent surgery with no transmitter insertion (sham-tagged), or served as controls also showed no significant differences in growth [7].
In contrast, other studies have found that acoustic transmitters decrease the growth rate of implanted fish. The growth of juvenile Atlantic salmon tagged with dummy acoustic transmitters has been reported to initially lag that of control and sham-tagged fish [8, 9], although in one study, tagged salmon eventually grew at the same rate as the other two groups [9].
There is a paucity of research examining the effects on growth and survival of rainbow trout after the implantation of acoustic transmitters. There is also a lack of research examining the growth and survival of acoustic-tagged fish when placed into an environment with untagged conspecifics, instead of having tagged and untagged fish placed into distinct rearing units. Thus, the objective of this study was to evaluate the growth and survival of acoustic tagged rainbow trout in a competitive environment, where tagged fish compete for food with untagged fish.
MATERIALS AND METHODOLOGY
Experimentation occurred at McNenny State Fish Hatchery, rural Spearfish, South Dakota, USA. Well water (11̊ C; total hardness as CaCO3, 360 mg/L; alkalinity as CaCO3, 210 mg/L; pH, 7.6; total dissolved solids, 390mg/L) was used throughout the study. Ninety Shasta-strain rainbow trout [mean (SD) initial length and weight = 277 (24) mm and 208 (60) g respectively] were assigned to one of three treatments, with 30 randomly-selected fish receiving each treatment. Tagged fish were anesthetized and received an inert acoustic transmitter. Sham fish were anesthetized, received an incision, but did not have a transmitter implanted. Control fish were anesthetized only. The 90 fish were divided into subsets of 30 fish each (10 tagged, 10 sham, and 10 control fish), with each subset placed into one of three hatchery raceways (4.7 m long x 2.4 m wide x 0.5 m deep). Rearing densities were extremely low and never even approached critical density index values [10]. Dissolved oxygen in all of the units was maintained at or above 8.0 mg/L.
Prior to surgery, fish were anesthetized using tricaine methanosulfate (MS-222; Argent Chemical Laboratories, Ferndale, Washington, USA), to stage 4 anesthesia [11]. After anesthesia, fish were measured to the nearest mm (total length), weighed to the nearest g, and tagged with a unique alphanumeric-coded visible implant (VI) tag (1.2 mm x 2.7 mm; Northwest Marine Technology, Inc., Seattle, Washington, USA) inserted in postorbital tissue to identify each trout throughout the duration of the study. After insertion of the VI tag, the trout in the tagged or sham groups were placed ventral side up in a grooved container. A 10 mm incision was made 3 mm from the midventral line, anterior to either of the pelvic fins. In the tagged group, an inert transmitter (9 x 24 mm, 3.6 g weight in air; VEMCO, Bedford, Nova Scotia, Canada) was inserted into the peritoneal cavity. Sham fish were anesthetized and incised, but no tag was inserted. Control fish only experienced anesthesia and handling. Incisions were closed using two simple uninterrupted sutures (Oasis Nylon Monofilament sutures 4-0, Glendora, California, USA).
The day after placement in the raceways, floating feed (4.5 mm floating Classic Trout, Skretting North America, Tooele, Utah, USA) was provided once per day well beyond satiation. Mortalities were removed daily, with the unique fish identifier (VI tag) and tag retention, if the fish was initially tagged, recorded. Total lengths (mm) and weights (g) were recorded 37, 65, and 90 days after the start of the experiment. In addition, at 90 days (the end of the study), liver, spleen, and viscera were removed and weighed to the nearest 0.0001 g. Individual fish weight gain, percent weight gain, length increase, percent length increase, Specific Growth Rate (SGR), and percent tag weight were also calculated. The following formulas were used:
Condition Factor (K) = [weight (g) / length (cm)3] x 100
Viscero-somatic index (VSI) = [(weight of viscera ÷ total fish weight) x 100]
Hepatosomatic index (HSI) = [(liver weight ÷ total fish weight) x 100]
Splenosomatic Index (SSI) = [(spleen weight ÷ total fish weight) x 100]
Weight gain = final weight – initial weight
Percent weight gain = (weight gain / initial weight) x 100
Length increase = final length – initial length
Percent length increase = (length increase / initial length) x 100
Specific growth rate (SGR) = [(Log final weight - Log initial weight) / time interval] x 100
Percent tag weight = (tag weight / initial weight) x 100
Statistical analysis was performed using the statistical programs R version 2.15.1 (R Development Core Team 2012) and SYSTAT 13 statistical software (SYSTAT, Evanston, Illinois USA). Logistical regression was used to compare initial weights and percent tag weight between surviving fish and mortalities using a max likelihood coefficient of determination (r2-maxL). Surviving fish for the study duration were labeled with a 0 while mortalities were given a 1. Logistical regression was also used to compare initial length to survival of the tagged treatment group. The effect of initial length on survival was evaluated using a Kolmogorov-Smirnov two sample test in an attempt to discern a minimum initial fish length for successful transmitter insertion. Two-way analysis of variance (ANOVA) was used, with tagging treatment and raceway as fixed factors. If a significant difference (P< 0.05) was observed, Tukey’s mean comparison procedure was conducted to determine differences among the means.
RESULTS
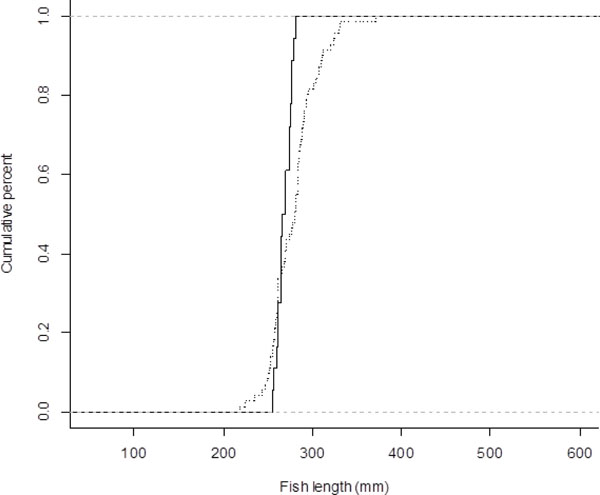
Twenty-two fish died during this study, with the majority of mortality (15 fish) occurring within the first week after tagging. Overall, four control fish, six sham fish, and twelve tagged fish died. Survival of fish used in this study was significantly affected by initial total length (P=.006, r2-maxL= 0.17) as fish that survived had a significantly larger distribution than fish that died (Kolmogorov-Smirnov test; D= 0.479, P=0.003; Fig. 1). Tagged individuals with an initial length greater than 280 mm all survived the duration of the study. However, tagged fish with an initial length of less than 280 mm had a survival rate of 59%. There was no significant effect of tag weight, as a percentage of initial fish weight, on tagged fish survival. There was also no significant effect of initial weight on fish survival as well. Eight tagged fish expelled their hydro acoustic tag during the study period. All eight of these fish survived to the end of the experiment.
Of the fish that survived over the 90 days of the study, significantly reduced growth was observed in the tagged fish in comparison to the control and sham fish. Weight gain (F8,2,2,4, P = 0.011), percent weight gain (F8,2,2,4, P < 0.001), length increase (F8,2,2,4, P = 0.001), percent length increase (F8,2,2,4, P = 0.001), and SGR (F8,2,2,4, P < 0.001) were all significantly lower in the tagged fish Table 1. There were no significant differences in any of the condition indices at the end of the experiment, except for a marginal significant increase in SSI in the tagged group (F8,2,2,4, P = 0.016). When determined by each sampling period, specific growth rates were only significantly lower (F8,2,2,4, P < 0.001) in the tagged fish after the first 37 days (Table 2). There were no significant differences among the treatments in SGR in the 38 to 65 day period or from days 66 to 90.
| Handled | Sham | Tagged | |
|---|---|---|---|
| Final length (mm) | 343 (6) | 352 (5) | 326 (7) |
| Final weight (g) | 537 (36) zy | 584 (28) z | 473 (41) y |
| Condition factor a | 0.128 (0.003) | 0.132 (0.003) | 0.131 (0.002) |
| Weight gain (g) | 344 (29) z | 343 (22) z | 243 (30) y |
| Percent weight gain (%) | 182 (13) z | 150 (10) z | 108 (12) y |
| Length increase (mm) | 71 (3) z | 62 (5) z | 45 (6) y |
| Percent length increase (%) | 26.3 (1.3) z | 21.8 (2.0) zy | 16.2 (2.1) y |
| Specific growth rateb | 0.487 (0.022) z | 0.431 (0.022) z | 0.340 (0.028) y |
| HSI c | 1.83 (0.09) | 1.88 (0.15) | 1.87 (0.09) |
| VSI d | 14.55 (0.49) | 15.53 (0.62) | 14.36 (0.72) |
| SSI e | 0.19 (0.01) zy | 0.17 (0.01) z | 0.24 (0.02) y |
| Mortality | 4 | 6 | 12 |
| Period | Handled | Sham | Tagged |
|---|---|---|---|
| Day 0 to 37 | 0.509 (0.055) z | 0.447 (0.022) z | 0.254 (0.032) y |
| Day 38 to 65 | 0.520 (0.038) | 0.439 (0.048) | 0.482 (0.019) |
| Day 66 to 90 | 0.419 (0.034) | 0.334 (0.054) | 0.406 (0.030) |
Weight gain (F8,2,2,4, P = 0.008), percent weight gain (F8,2,2,4, P = 0.007), length increase (F8,2,2,4, P = 0.006), percent length increase (F8,2,2,4, P = 0.014) and SGR (F8,2,2,4, P = 0.002) were significantly different among the three raceways (Table 3). However, the pattern was consistent in each raceway, with tagged fish experiencing decreased growth initially compared to the other two treatments.
| A | B | C | |
|---|---|---|---|
| Weight gain (g) | 330 (24) zy | 359 (32) z | 243 (31) y |
| Percent weight gain (%) | 106 (15) z | 124 (16) y | 107 (17) z |
| Length increase (mm) | 63 (26) z | 69 (21) z | 48 (22) y |
| Percent length increase (%) | 62.9 (5.2) zy | 69.1 (4.6) y | 49.2 (5.2) z |
| SGR | 0.443 (0.019) z | 0.470 (0.025) z | 0.362 (0.031) y |
| Mortality | 8 | 9 | 6 |
DISCUSSION
It is apparent that the rainbow trout in this study were affected by the surgical insertion of acoustic transmitters. The initial lag in growth from rainbow trout implanted with transmitters is similar to those reported with other salmonid species [9]. Decreased growth in tagged Atlantic salmon, compared to controls, ranged from 9 to 36 days post-tagging [8]. Similarly, the growth rates of juvenile Chinook salmon with surgically implanted radio tags were reported to be slightly impaired in the first three weeks after surgery, but growth was comparable after eight weeks to those of control fish [5]. In contrast, other studies report that the growth of fish after receiving acoustic transmitters is not significantly different than that of control or sham fish, but there are differences in the methodology used in those studies compared to the current experiment. In studies with Chinook or sockeye salmon, no differences in fork length or mass were observed among fish that were implanted with acoustic transmitters, underwent surgery with no transmitter insertion (sham-tagged), or served as controls [6]. However, those fish that were much smaller than those used in this study, and growth was only evaluated 21 days post-tagging [6]. In another study with Chinook salmon, no differences in growth between acoustic-tagged Chinook salmon and a sham group were observed, but sampling at the end of 160 days may have masked any early reductions in growth [1].
Based on the results of this study, it is essential that rainbow trout implanted with acoustic transmitters be allowed to sufficiently recover from the surgery and adapt to the inserted transmitters so that they can accurately represent the movement, behavior, and survival of the untagged fish [2-4]. A minimum of 38 days post-tagging is necessary for the implanted fish to grow similarly to untagged conspecifics. The subsequent recovery in growth of the tagged fish after 38 days indicates that at least for the length of this study, there were no longer-term ill effects of tag residence within the body cavity. While there is no data to conclusively say if these results are applicable to other internal tag types, we suspect that the placement of any foreign object, such as radio tags, within the body cavity would likely elicit a similar response.
The 40% mortality in tagged fish in this study was double the previously reported in tagged age-one Chinook salmon [1]. This high mortality in the tagged fish is likely due to a combination of factors. Success of acoustic transmitter implantation may be dependent on the skill of the surgeon [12]. The individual performing the surgery for this study was relatively inexperienced and had not received significant feedback, which can be extremely beneficial [12]. In addition, the elevated mortality of trout in all three of the treatments may indicate issues with the condition of the rainbow trout [6]. The timing of mortality within the first week after surgery observed in this study follows a previous pattern of acoustic tag post-implantation mortality [9].
Similar to this study, other authors have identified thresholds in fish size for the survival of salmonids implanted with acoustic tags. Our study found that fish were not limited to the “2% rule” of tag weight to body weight ratio [13]. Other authors have determined various thresholds that are not only tag type dependent, but also species dependent. An apparent minimum tag to body weight ratio for juvenile Chinook salmon implanted with acoustic transmitters has also been observed, with fish with a ratio of greater than 8.2% negatively affected by tag implantation [14]. A threshold of 5.8% transmitter-to-body-weight ratio in juvenile Chinook salmon has also been reported, with 100% mortality occurring above this threshold for ultrasonic transmitters [15]. With 1-year old Chinook salmon, a 5.6% transmitter-to-body-weight ratio was previously reported as appropriate [1], with a 7 to 8% body weight ratio in coho salmon inserted with three different sizes of hydro acoustic transmitters also recommended [16]. Because of differences in suggested body-weight-to-tag-weight ratios, telemetry studies should be validated by laboratory trials prior to field application. The least burdensome transmitter that can satisfy the needs of the study should be used [1].
Acoustic transmitters should be retained long enough for data collection [3]. Although several of the trout in this study expelled their inert transmitters relatively soon after surgery, retention time was at least 90 days for most of the tagged fish. Tag shedding may occur because of the expulsion of the tag through the incision [17, 18], expulsion of the tag through the body wall [17, 19], or loss through the intestine [17, 20]. Because we sampled fish in 30 d increments, we were able to observe individuals regularly and believe that the majority of fish that expelled their tags in our study passed them through the body wall based on tag bulge and inflammation. One other study concluded that tag loss occurring before 31 d was likely occurring at the incision site and all tag shedding after was attributed to being passed through the body wall [21]. The size of fish may play a role in transmitter retention [1], and other factors, such as suture types and surgeon skill, also influence tag retention [1, 12]. The relatively low water temperatures of 11°C used in this study should have aided tag retention [1].
The splenosomatic index is an indirect measurement of immune function in fish [22], and dramatically increases in stressed or diseased Rainbow Trout [23]. The splenosomatic values observed in this study were all slightly higher than those reported for rainbow trout in a number of other studies [24-27], but were very similar to those observed in one other study [28, 29], and well within normal values [30]. Thus, the small, but significant difference, in splenosomatic index among the treatments was likely not biologically significant.
The similar HSI and VSI values among the treatments at the end of the study indicate that the tagged fish, although smaller than the untagged fish, were still partitioning nutrients in the same manner. Both VSI and HSI are indicators of lipid deposition [31-34], and HSI is particularly reflective of nutritional status [35]. Similar HSI values among the treatments may also suggest similar physiological stress levels [36] at the end of the study.
Why the fish, regardless of treatment, had a slower growth rate in one of the raceways is unknown. It is possible that there was a positional effect, because the slower growth raceway was the only one that bordered a hatchery road. However, all of the raceways were completely covered, which should have limited any exposure to disturbances. In addition, no positional effects have ever been observed in prior studies or during hatchery production. It is more likely that the difference was due to possible differences in how the fish were handled initially. The slower growth raceway fish were the first to undergo anesthesia or surgery, and fish placed in subsequent raceways may have benefitted from the experience gained by the tagging crew [11]. More importantly, the lack of an adequate explanation for the raceway differences in growth does not impact the differences in growth among the treatments, because the treatment results were consistent among all three raceways.
CONCLUSION
Rainbow trout inserted with acoustic transmitters should be held for a minimum of 38 days to ensure that their subsequent behavior and fate is similar to untagged conspecifics [4]. A minimum total length of 280 mm is also required to maximize Rainbow Trout survival when implanted with the 3.6 g, 9 x 24 mm, transmitters.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENT
We thank Greg Simpson, Patrick Nero, Eric Krebs, Jeremy Kientz, and Kelby Torgerson for their assistance with this study.