REVIEW ARTICLE
An Overview of the Current Status of Lake Naivasha Fishery: Challenges and Management Strategies
James Njiru1, *, Edna Waithaka2, Peninah A. Aloo3
Article Information
Identifiers and Pagination:
Year: 2017Volume: 10
First Page: 1
Last Page: 11
Publisher Id: TOFISHSJ-10-1
DOI: 10.2174/1874401X01710010001
Article History:
Received Date: 28/09/2016Revision Received Date: 01/12/2016
Acceptance Date: 22/12/2016
Electronic publication date: 31/03/2017
Collection year: 2017
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Lake Naivasha a shallow, freshwater body and a Ramsar site is found in the eastern arm of the Kenyan Rift Valley. This paper used published, unpublished and analyzed data to assess the status, challenges and management options for the fishery. Lake Naivasha fishery is based on exotic species that fluctuates depending on fishing regime, lake water level and aquatic plant concentrations. The fishery has been dominated by different species with the current catch contribution consisting mainly of common carp, Cyprinus carpio, Nile tilapia, Oreochromis niloticus, blue-spotted tilapia, O. leucostictus and African catfish, Clarias gariepinus. The minimum and maximum catch of 21 t yr-1 and 1181 t yr-1 was reported in 1997 and 2015, respectively. The main threats to the lake fishery are anthropogenic influences emanating from within the lake and its basin. The factors include intense fishing, exotic species introductions, water abstraction, lake level fluctuations, wetland utilization, eutrophication, and land degradation. There are also several conflicts of interest amongst the stakeholders in agriculture, fisheries, wildlife, tourism, conservation and geothermal electricity generation. There is fear that if the current trend persists, the lake and its fishery may be headed for extinction. The management measures instituted in the lake do not seem to have arrested reduction in fish catches nor reversed deterioration in water quality. For sustainable utilization of Lake Naivasha and its fishery, there is a need to consider a holistic ecosystem approach of the basin management. Additionally, all the relevant stakeholders should be involved in formulation and implementation of the decisions to manage the fishery.
INTRODUCTION
Lake Naivasha, a freshwater body is located about 100 km North of Nairobi in the Eastern Rift Valley of Kenya. The shallow endorheic lake has a mean depth of 3.5 m and a maximum of 8 m [1-3]. The lake basin lies at an altitude of about 1890 m above sea level, receives about 80% and 20% of its water from Rivers Malewa and Gilgil, respectively [4]. The area surrounding the lake is semi-arid with an average annual rainfall of 1,350 mm in the mountains to 600 mm on the shores of the lake. The lake is bordered by a stripe of varying width of Nile grass, Cyperus papyrus L. wetland and submerged macrophytes with the main species being water nymphs, Najas pectinata (Parl.) Magnus. The lake has floating mats of Kariba weed, Salvinia molesta Mitch, and water hyacinth Eichhornia crassipes (Mart.) Solms. The location and characteristics of Lake Naivasha could be found in a map by Oyugi [5].
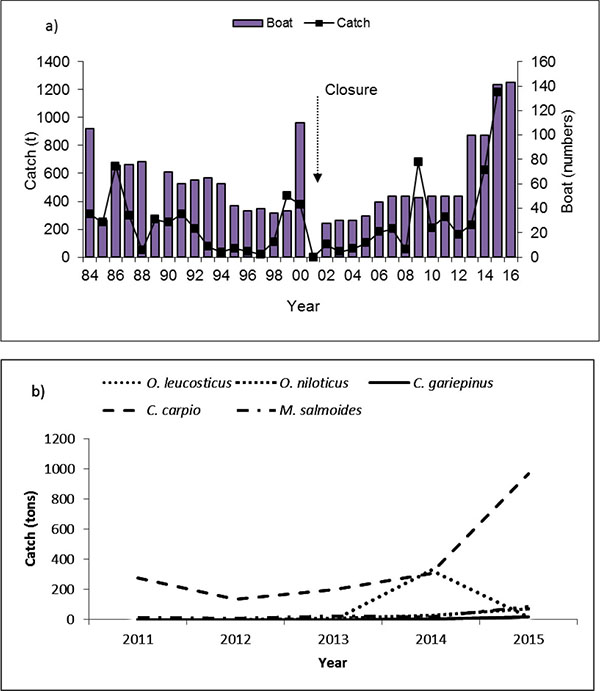
Fish catch composition of Lake Navaisha consists of introduced species that vary over time [6-8]. A summary of introductions since 1925 and their status is presented in Table 1. The commercial fishery of Lake Naivasha started in 1959 using gill nets for tilapias, while rod and line were used in sport fishery to catch bass. In an attempt to reduce fishing pressure and resuscitate the dwindling catches of Macropterus salmoides and Tilapia zillii, fishing ban was enforced in 2001 [9]. Thereafter, a closure was imposed from 1st June – 1st October starting in 2003. The fishery was re-opened with maximum allowable boats of 43 from previous numbers of 110 (Fig. 1a). Further, boats were allowed to have only three crew members and 10 gillnets of 4" per fishing trip. Sport fishers using rod were allowed a maximum of 5 fish day-1. In addition, those trading in fish were required to have daily fish movement passes from the beaches to markets.
| English name | Scientific name | Introduction date | Current status |
|---|---|---|---|
| Black lampeye | Aplocheilichthys antinorii (Vinciguerra, 1883) | Last reported in 1962 | Endemic: Probably extinct by M. salmoides predation |
| Straight fin barb | Enteromius paludinosus (Peters,1852), Synonym Barbus paludinosus | Came through in-flowing rivers recorded since 1982 | Currently occasionally caught |
| Guppy | Poecilia reticulata (Peters, 1859) | Date unknown | Recorded since 1982 Currently occasionally caught |
| Black bass | Micropterus salmoides (Lacépède, 1802) | 1929 as sport fishery, re-introduced in 1946/1951 | Found today |
| Sabaki tilapia | Oreochromis spirulus niger (Günther, 1894) | 1925 | Last caught1971 |
| Redbelly | Coptodon zillii (Gervais, 1884) previous known as Tilapia zillii | 1959 | Found today |
| Blue-spotted tilapia | Oreochromis leucostictus (Trewevas, 1933) | 1959 unintentionally with T. zillii | Found today |
| Blue-spotted tilapia/ Sabaki tilapia | O. leucostictus x O.s. niger hybrid | Plentiful in 1960s | Last caught in 1972: lost due back crossing with O. leucostictus |
| Nile tilapia | Oreochromis niloticus L. | 1967. Vanished by 1971, probably due to young predation by bass. | Re-introduced in 2011. Currently present |
| Mosquito fish | Gambusia sp. | Date unknown | Absent since 1977 |
| Rainbow trout | Oncorhyncus mykiss (Walbaum, 1792) | Date unknown. Came through River Malewa from fish farms | Occasionally caught |
| Common carp | Cyprinus carpio L., sub species mirror carp C. carpio specularis and leather carp, C. carpio coiaceus | Recorded in 2001. Accidental introduction through inflowing rivers from fish farms | Found today |
| African catfish | Clarias gariepinus (Burchell, 1822) | Accidental introduction: escaped from fish farms into inflow rivers. | First recorded in 2012. Currently present |
| Louisiana crayfish | Procambarus clarkii (Girard, 1852) | Introduced in 1950s. to provide food for Bass | Currently present |
Threats to the lake fishery revolve around unsustainable resource exploitation both within the lake and its catchments [10]. These include fish introductions and accidental arrivals, pollution from agricultural activities, sewage waste, siltation, habitat degradation, illegal fishing, fluctuations in Lake level, floral distribution and water abstraction. The lake became a Ramsar site in April 1995 [11], but this does not seem to have slowed down pressure on the lake’s ecosystem and the fishery [12]. This paper traces the trends and status of Lake Naivasha fishery, its challenges and suggests management strategies that may enhance sustainable utilization. The study used a combination of literature review and analysis of new data provided by the Kenya Marine and Fisheries Research Institute (KMFRI) and State Department of Fisheries and the Blue Economy (SDF & BE), Kenya.
CATCH TRENDS
Lake Naivasha has had great variations in the total amount of fish caught over the years. The highest and the lowest documented annual catch was of 1150 t yr-1 and 21t yr-1 in 1970 and 1997, respectively [7], raising to 1181 t yr-1 in 2015 (Fig. 1a). The fishery development can be divided into three phases: a) boom and bust (1963-1977) with average annual catch of 488 t (average 269–706 t), b) stable (1978–1987) with a catch of 387 t (average 257–517 t), and c) poorly performing fishery (1987–2001) with catch of 155 t (average 74–236 t) [6].
Fishery statistics show that between 1987 - 2000 the fishery was dominated by tilapias, Oreochromis leucostictus (71.7%), T. zillii (8.8%) and M. salmoides (19.5%) [7]. Between 2002 to 2006, there was a change in contribution with a shift to Cyprinus carpio (51%), O. leucostictus (21.9%), M. salmoides (13.2%) and T. zillii (1.5%) [14]. In the year 2007 and 2008, the catches were dominated by C. carpio (81.7%), O. leucostictus (9.7%), M. salmoides (8.3%) and T. zillii (0.3%). In 2015, the fishery was dominated by C. carpio, O. niloticus, O. leucostictus and Clarias gariepinus with contribution of 83.4%, 7.3%, 6.0% and 19% and 1.7% species, respectively (Fig. 1b).
Earlier studies in Lake Naivasha fishery estimated maximum sustainable yield (MSY) to exceed 400 t yr-1 from the initial 140 t yr-1 if the stocks were properly managed [6, 15]. Based on the potential of increasing yield in the lake, a proposal was made to have more introductions from the African fauna. To sustain the yield, the new entrances to the lake were to be linked to enhance management and conservation strategies. On the basis of food availability in the lake, there were proposals to introduce a bottom feeder such as Mormyrus sp. to consume the under-utilized benthic fauna of oligochaetes and chironomid larvae [16]. The proposal was adopted by the riparian stakeholders that were led by Lake Naivasha Riparian Association (LNRA) but no introductions were done during this period. However, in 2001, the Government of Kenya through the Department of Fisheries reintroduced Nile tilapia in the lake to enhance the stocks.
There has been beneficial and adverse impacts on the interactions between the multiple exotic species in the lake and this may probably be best demonstrated by the C. carp. The carp improved the lake fishery from near collapse in 2001. However, its dominance has had inherent adverse effects on the other species [14]. The dominance of C. carpio and the reduction of the other species in the lake may be attributed to changes in water quality and the ecosystem. The carp is a very resilient species and can withstand degraded habitats [17]. Adverse effect of C. carpio to other species is mostly due to its feeding behavior [7]. The carp feeds on benthic organisms by taking in sediments with food items and retains the prey while expelling the sediments through the gills [18].
Carp feeding mode uproots aquatic plants, stirs the sediments at the bottom, increases water suspended solids and affects water turbidity [18]. Uprooting of submerged macrophytes compounds a similar damaging impact by Procambarus clarkii in the lake [8]. Loss of macrophytes further increases re-suspension of sediments through wind driven circulation. Increased turbidity decreases light penetration that is important for photosynthesis of submergent plants and phytoplankton. Decreased phytoplankton and aquatic plants reduces food-base for phytoplanktivore fishes such as O. leucosticus and herbivorous T. zillii. Further, upsurge in turbidity affects the feeding ability of M. salmoides as a visual feeder.
By disturbing the lake bottom C. carpio affects reproduction of T. zillii that lays sticky eggs on substratum such as pebbles, sand and vegetation [7]. Common carp and T. zillii compete for substratum space to lay their adhesive eggs, with the carp having a competitive advantage because it is not selective on the substratum type [19]. Feeding style of stirring the bottom by C. carpio also affects reproduction of other species such M. salmoides, O. niloticus and O. leucostictus that construct their nest on the lake bottom. Although no direct predation of adult fish by the carp has been documented, its omnivorous behavior leads to consumption of juveniles and eggs of other species in the lake. Such behavior may upset establishment and recruitment of the affected species. Nonetheless, the greatest impact on Lake Naivasha fishery may stem from anthropogenic impacts and management strategies instituted.
CHALLENGES
Illegal Gear and Fishing
The annual closed fishing season introduced in 2001 and use of gears of 4" was meant to control excessive fishing effort, not to capture immature fish and to allow fish stocks to recover [14]. The fishery was reopened in 2002 with a five months closure fishing period every year since 2003. However, in 2013, the closed fishing period was lifted by the County Government of Nakuru and all the illegal fishers and poachers were incorporated in what was thought to be a move to reduce illegal fishing. This initiative seems to have backfired with new poachers entering the fishery and raising further the number of legal and illegal fishers in the lake; bringing the sustainability of the fishery into question. We observed fishers using illegal gears such as seine and monofilament nets, while passive gillnets were used as active gears (used as seines). There was also use of gillnets of 3.5" and below to target the smaller sized O. niloticus, O. leucosticus and T. zillii. This rendered C. carpio as a by-catch of the tilapia fishery leading to the capture of immature carp. Experimental gill netting studies revealed that gears below 4" mainly captured fish below size at first maturity (Fig. 2). The fishers regularly used illegal gears to fish in shallow areas that act as breeding and nursery grounds for most fishes in the lake. We also observed fishers targeting the bigger brooder specimen of the C. carpio using gillnets of 8" to 10". The use of undersize nets that catch juvenile fishes and large sized nets increases fishing effort than may be permitted, while seining by illegal fishers had the potential to affect the performance and sustainability of the fishery [14, 15]. Additionally, illegal fishers and fishing are a serious threat to conservation efforts instituted by the Kenyan government.
ANTHROPOGENIC STRESSORS
Human Population
The growth of population around Lake Naivasha is around 3.1% annually and is concentrated in the urban areas with mean density of about 500 persons per km2. This population increased up to tenfold over the last three decades from around 7,000 in 1969 to around 67,000 in 2002 in the urban areas [20]. Following the 2009 census, Lake Navaisha had approximately 650, 000 persons of which around 160,000 lived along the lake [21]. Increase in human population has continued to put more pressure on waste disposal systems posing serious threats to the health of the lake and its fishery. This is because most of the Naivasha town is not served with an operational sewerage system. This allows raw sewage to find its way into the lake and contributes to water pollution.
Rapid growth in population and need for more energy has increased degradation of the lake catchment with the cutting down of trees to provide for firewood, charcoal and timber for construction [22]. The forest clearance has led to increased soil erosion and transport of nutrients into the lake [10]. High volumes of fertilizers, pesticides and effluents produced by the Naivasha floricultural industry have been blamed for the large rise in Pb, Cd and Cu levels observed in the waters of Lake Naivasha [23, 24]. These pollutants have been found in high concentrations in fish tissues and have accumulation effects on the food chain [24]. The decline in African fish eagle around the lake is attributed to build up of pollutants in fish they consume [25].
Loss of Wetlands
Greatest part of the land surrounding Lake Naivasha was previously protected by a border of C. papyrus wetlands that controlled the ecology of the lake by regulating entry of nutrients, sediments and acted as natural purifiers of water [26, 27]. The swamp that covered about 1,200 ha has been reduced to less than 200 ha, compromising its efficiency and effectiveness as a buffer area allowing entry of nutrients into the lake that contributes to the lake eutrophication [28]. Reduction of the swamp is attributed to direct human clearance, lowering of the lake waters, grazing by herbivores such zebra, buffalos, cattle, and P. clarkii [12, 27]. The wetland degradation and subsequent eutrophication of the lake further reduces suitable areas for fish to breed and replenish the declining stocks.
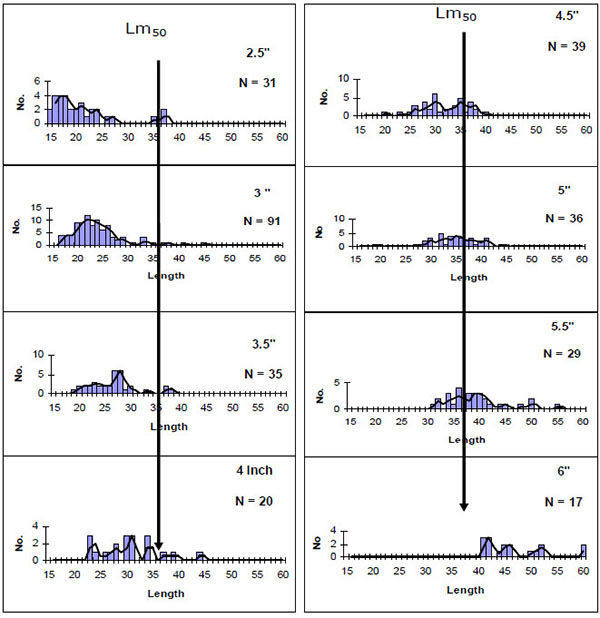 |
Fig. (2). Size selections of C. carpio by different mesh sizes. Arrows indicate the point at which 50% of the fish are sexually mature (Data source: KMFRI 2007). |
Water Abstraction
Changes in Lake Naivasha water level is attributed mostly to the amount of water used, rainfall, evaporation, and underground seepage. Studies have shown that 80% reduction in water levels is associated with the use of water to irrigate horticultural farms [29]. It is estimated that around 3 to 5 x 106 m3 of water is abstracted every month, though this may be more because the water used is not metered [22]. In the 1980s, the over-abstraction of water from the whole basin led to water reduction up to 3 m lower [4]. The lake levels reduction may also be related to deforestation in the catchment area which has led to lower amount of rainfall. It is estimated that if abstraction continues at the current rate, the lake may be turned into a pool of water barely 30 km2 [28]. Such a change will have profound effects on the marginal swamps leading to their reduction and subsequent increase in nutrients entry into the lake, an upsurge in algal productivity, a compromised ecosystem that would adversely affect the fishery [27].
Water Quality
Lake Naivasha is currently more eutrophic than in the 1970s [13]. There has been a dramatic elevation in chlorophyll-a concentration in the lake attributed to increased nutrients loading into the lake [30]. Recent studies found algal biomass, measured as chlorophyll-a concentration ranged between 16, 850 μg l−1 and 37, 730 μg l−1 with a mean concentration of 2,455 ± 7,170 μg l−1 (KMFRI unpublished data) compared to 40 - 50 μg l−1 in the 1980s [13]. The current total nitrogen of 4 mg l−1 compared to 0.04 mg l−1 in the 1970s placed Lake Naivasha as eutrophic and L. Oloidien as hyper-trophic [10, 26]. The lake posted Dissolved Oxygen (DO) of up to 9 mg l-1 in the late 1990s compared to an average of 7 mg l-1 in 2015. The lake experienced more hypoxic conditions with deep waters having temporal stratification especially during calm days with DO levels less than 3.0 mg l-1 below 4 m (Fig. 3), that may be unsuitable for survival of most fish (KMFRI unpublished data). Recently fish death in the lake were associated with increased phytoplankton production and decreased DO levels in the lake [31].
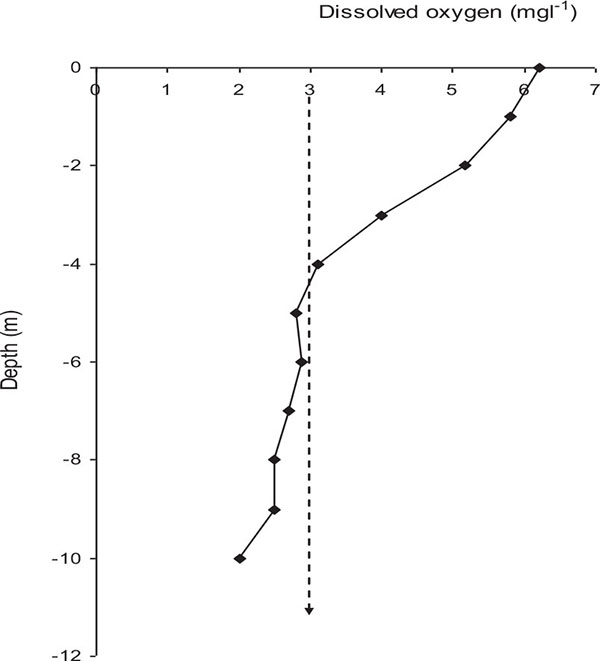 |
Fig. (3). A typical example of oxygen profile in deeper waters of Lake Naivasha, Kenya. Left of the arrow indicates water in which most fish will not dwell (Data source: KMFRI 2015). |
The lake had a high transparency ranged between 3.5 to 4.5 m up until the 1970s [31]. Recent studies put average transparency at 0.88 m in the open lake. The reduced transparency since 1970 is a combination of loss of macrophytes [32], and increased algal blooms resulting from increased eutrophication [10, 28]. The benthic foraging behavior of C. carpio has exacerbated the turbidity problem, and reduces the prospects of return to the former clear waters prior to 1970s [32]. Reduced transparency may have an effect on the feeding success of visual feeders such as M. salmoides.
MANAGEMENT STRATEGIES
Institutional Mechanism
Lake Naivasha Riparian Association
The riparian land around Lake Naivasha is under the custodian of a Non-Governmental Organization (NGO), the Lake Naivasha Riparian Association (LNRA) that works through a committee representing stakeholders with an interest in the lake [33]. The goal of the committee is to achieve sustainable utilization of the lake resources, including the fisheries. The association was instrumental in lobbying for the lake to achieve its Ramsar status in 1995. It has also developed a Lake Naivasha Management Plan and a strategy for its implementation. The association has gathered scientific information on the lake and made suggestions on how best to manage and utilize the lake resources. However, even with a well thought out management plan, success has eluded LNRA in its goal to achieve sustainable utilization and conservation of Lake Naivasha and its fisheries. Notably, the major drawback is the lack of power for enforcement because its approach on the Codes of Conduct is voluntary and is not anchored in law. Due to this, non-compliance by the users on the agreed upon measures is high. For more success, the association should think of having by-laws and penalties agreed upon by the members. Offenders could then be punished using the agreed upon regulations.
Imarisha
“Imarisha” is a Kiswahili word which literary means “to make stable”. Imarisha program is a Public-Private Partnership (PPP) initiative to mitigate destruction occurring around Lake Naivasha Basin, safeguard sustainability development, secure investments and improve community livelihoods [http://www. Imarisha naivasha.or.ke]. The anticipated outcomes of these initiatives include: “wise use” of Lake Naivasha resources and its riparian zone. Imarisha Naivasha has initiated several projects in collaboration with their partners towards realizing its mandate. These include improvement of fisheries infrastructure such as landing sites through Beach Management Units (BMUs). Imarisha Naivasha Water stewardship Project (INWaSP) objective is to restore the lake basin through programs such as reforestation in order to reduce erosion and nutrients loading into the lake so as to improve the lake’s health and maintain a conducive environment for the fisheries.
Beach Management Units (BMUs)
Management of fisheries in Kenya has been a top-down approach led by the central government with little involvement of stakeholders [9, 34]. It was recognized that for better management of Lake Naivasha fisheries, a participatory management approach that is coupled with law enforcement may yield better results [9]. It is in this context that the Kenyan government changed the management strategy and brought in stakeholders through community involvement in Lake Naivasha by forming the Beach Management Units (BMUs). In Lake Naivasha, the BMUs were formed in 2001 following the closure of fishing after near collapse of the fishery [9]. Community involvement in the management of Lake Naivasha fisheries saw a general improvement in fish catches [9]. For example, in January 2001, an average catch from a boat was 44 kg using unlimited number of fishing gears. Four years later, after formation of BMUs and use of the recommended 10 fishing gill nets per boat, the catches rose to 310 kg per boat. In the same time period, total annual fish production rose from 5,000 kg to 13,942 kg with a value of USD 2,000 and USD 6,000, respectively. It was also observed that legal fishers were using the recommended gears of 4 inches. However, the success of co-management was short-lived because fishers that were removed from the fishery returned to use legal and illegal gears due to increased riparian population and limited sources of alternative livelihoods. Furthermore, the political class felt the closure excluded their voters accessing the fishery and their pressure led to lifting of the fishing ban in 2013, making the fishery an open access resource once again.
Nonetheless, all is not lost; co-management experience from the lake may be regarded as a learning process and can be improved [9]. Thus, there is a need to revisit the process and pick the lessons learnt before launching an improved process once again. Nunan [34] argued that co-management structures and systems have the capacity to be more responsive and flexible, but they require more support, technical and financial, and acceptance of adaptive governance. She further contends that co-management is a process, rather than a static arrangement which is supposed to yield results immediately. Co-management process is therefore dynamic and can only evolve in practice over time with the support of stakeholders and the government.
RECOMMENDATIONS
Wetland Restoration
For sustainability of Lake Naivasha and its resources, wetland restoration holds a key role [30]. Major interventions for restoration would involve considering ecohydrology at the site where River Malewa enters the lake or use of ecological manipulation around Gilgil River. The two approaches are believed to be ecologically achievable but the authors believe Gilgil River restoration procedure would be a better option. The major limitation to the success of any of the two projects would be related to human interference. Moreover, planting of more trees could convert floricultural land to forested land and this would increase riparian land under vegetation. Plant cover would subsequently reduce the pollutants entering the lake by regulating water filtration and flow. Regulated water flow and decreased pressure on land may allow papyrus wetland to recover further, thus, reducing contaminants entering the lake leading to an improved water quality that would be more suitable for a sustainable fishery [4]. For the success of these projects, it will be prudent to involve the communities from the inception to the implementation phases. This will enable the communities to own the projects and appreciate their benefits thereafter.
Sustainable Development
Rising populations and demand for natural resources implies that the challenges of sustainable development are unavoidable for Lake Naivasha and its basin. These challenges will be felt more in the vulnerable yet highly productive areas such as the fringing wetland areas of the lake that are key to the sustainability of the fisheries [27]. For Lake Naivasha to have been designated a Ramsar site, one of the conditions was to formulate wetland management strategies to allow their wise use for the benefit of mankind while maintaining its natural ecosystem properties. Sustainable utilization and development plans of the lake fisheries require the management regimes to use a holistic approach that encompasses the entire catchment and includes all possible factors that may affect the health of the lake ecosystem. Decisions should be made starting from the local to the national level for the solutions to be more accepted by the stakeholders [26]. For example, there could be concerted efforts by the relevant authorities to promote efficient farming practices that will conserve water, guarantee safe use and disposal of agro-chemicals. These measures would ensure sustainable use of water at the same time reduce environmental pollution from agricultural activities.
Water Abstraction
Small users of water around Lake Naivasha region operate at a 31% water use efficiency in their irrigation systems [31]. This inefficiency contributes considerably to decreasing lake water level and leads to increased pressure on the surrounding ecosystem and the fishery. Improved water efficiency utilization may be achieved by use of appropriate irrigation methods and planting of suitable crops. Secondly, all the water abstracted from the lake needs to be metered to enable determination of optimum abstraction amounts. Enforcement of the Water Act No. 8 [35] among other supplementary regulations may also improve conservation of the water. For example, the Act permits abstraction only at flood flow time and requires users to have a 90 days storage period. This measure is meant to allow storage of water during rainy seasons therefore it is used in drier months. To make this regulation more effective, there is a need to strengthen its operation since those abstracting water for irrigation have not installed the required storage facilities. Additionally, there is need to set limits on the permits given because currently they do not specify the amount of water to be abstracted. To improve water management, there is need to strengthen the weak inter-linkages in water related sectors. There are several and at times conflicting laws in management of the water sector. For instance, the Environmental Management and Coordination Act (EMCA) and the Water Act have mandate to manage the Lake Naivasha Basin and each claims superiority over the other. To compound the matter more, the leadership of Naivasha town seems not to be in support of Lake Naivasha Management Plan and its implementation [22]. Thus, there is a need for a concerted effort by the government through the relevant ministries to harmonize frameworks and working relationships in and around the Lake Naivasha for better coordination and conservation of the lake.
Aquaculture
Lake Naivasha Basin can provide good prospects for aquaculture growth due to many small public water bodies and warm weather conditions (average of 25°0C) that are suitable for most fish growth [36]. Additionally, the region has ready market due to its high human population. Increased fish production can support livelihoods, increase food security and reduce fishing pressure on Lake Naivasha. The lake itself is also suitable for water based aquaculture where cages and pens may be used [36]. In the lake, pen culture seems the most viable option because of its shallowness. Cage culture would require deeper waters and may raise more conflict among fishers. Pen culture though a better option, has higher initial costs but the expenses thereafter are low. Pen culture in the papyrus swamps along the lake may serve as buffer for sewage water as it is done presently in the Asian countries with similar aquaculture practices. The method would also effectively use open waters to increase fish production. This aquaculture system would also allow unemployed, landless and fishers to join fish farming. The main draw-back in the establishment of pen culture in Lake Naivasha is access to the lake water because most of the shoreline is privately owned. For this venture to succeed, the LNRA has to grant permission to use the riparian land. To achieve positive contribution of aquaculture, the government should also avail means for getting fingerlings, appropriate and affordable fish feeds, credit facilities, training, outreach and information to farmers.
Tourism
Kenya receives about 1.5 million international visitors annually of which approximately 5% passes through Lake Naivasha region [37]. The total contribution of tourism to Naivasha was valued at KSh 600 million (USD 6 million) [38]. Lake Naivasha potential for tourism development is enormous owing to its proximity to Nairobi, the capital city of Kenya, and due to its more secure environs. After recent terrorist attacks at the coastal of Kenya and Nairobi, many tourists now prefer visiting Lake Naivasha region [37]. The acacia forested and herbivore infested lake shores, together with wetlands along the shores provide an excellent aesthetic value for sightseers [28, 37]. There are over 350 bird species in and around Lake Naivasha, including fish eagles and kingfishers that are dependent upon the fishes of the Lake. The unique bird life is a major tourist attraction and generates the much needed employment within the hotel industry and its associated activities [27]. Tourists also flock the lake for leisure fishing where they target M. salmoides. Most of the sport fishers are indigenous visitors from Nairobi, the Kenyan capital, looking for a getaway from the bustle and hustle of the city life. Increased tourism around Lake Naivasha will aid in conservation of its variety of aquatic flora and fauna.
However, tourism has its downslide, when the business takes the neo-colonial turn with only a few foreign individuals controlling the major part of the investment [39]. From this arrangement, most of the profit ends up outside the basin and local communities remain marginalized in the presence of plenty. Tourism ignites social evils such as juvenile prostitution, school dropouts and drug addiction. Additionally, over dependence on a fragile sector like tourism may not be good for a local economy. A slight disturbance on security or an outbreak of an infectious disease can easily threaten the entire sector [40]. To maximize tourist leisure, accommodation is often erected near areas with concentrated wildlife and at times near breeding grounds which may interfere with the ecological integrity of such an ecosystem [41]. Some lodges lack proper sewage system and discharges waste direct into the lake negatively affecting water quality.
Sport Fishing
Sport fishing may lead to growth of cottage industries, and create jobs for the local communities including possible poachers [42]. It has also been found that the presence of sport fishers keep poachers at bay. Their impact on the fishery is not like extractive fishing, and they also provide data on species status in the ecosystem that may influence policy on management of a fishery. Funds from angling can support education and conservation projects. In India, anglers organize camps for children where they are exposed to species available in the lake and their importance [42]. This can be extended to Lake Naivasha to make the young people to be aware of their resources and the need to conserve. Recreational fishing, a popular leisure activity, can potentially support conservation of species and provide socio-economic benefits to local economies [43]. It should be noted that poorly managed sport fishery without well-defined rules may harm the same fishery it is supposed to protect. Sport fishers may introduce exotic species through life baits. Also, they may discard fishing lines and interfere with the ecosystem by removing riparian plants in order to reach better sites for fishing. To control this negative impact, the government can enact policies to support and regulate the sport fishery.
Fisheries Management and Development Act
In 2016, the government enacted a new law, the Fisheries Management and Development Act, repealing the older Fisheries Act (Cap 378) and Fisheries Protection Act (Cap 380) [44]. The new law takes into account the new dispensation in Kenya governance structure following the enactment of a new constitution in 2010 and creation of national and 47 county governments. The law outlines the role that the national and county governments have in development and management of fishery resources. The main objective of this Act is wise use of fishery resources while protecting the environment, improve livelihoods of persons depending on fishing and increase food security. Unlike the previous Act, management of the fishery is now more focused and has been brought under one roof headed by a Director-General. The Act is more progressive and encompasses disciplined forces in fisheries management. Furthermore, it has inter-agency Monitoring and Control surveillance. It is believed that this approach will be more effective than in the previous scenario where the fisheries sector acted independently of the law enforcing agencies.
Information
To sustainably utilize Lake Naivasha and its resources, more information on the interaction between the lake and its catchment is crucial. A better understanding of the synergies between the rivers, land-use patterns in the catchment and the lake resources would be key in formulating polices for sustainable development and management. Monitoring the interactions between water quality and fish stocks would be vital in understanding dynamics of the fishery. This would emanate from quality data on hydrographical factors, fish catches, biological and ecological parameters in the lake. Fishing pressure has been heavily blamed for the demise and decline of the lake fisheries. Such vital information as good fish catch statistics, biological parameters, and indices of the distribution and abundances of commercial fish species, which are needed for defining management policies, should be sought. Increased local stakeholders involvement and creation of awareness through extension, and educational workshops would increase availability of information [45].
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
We are grateful to State Department of Fisheries and the Blue Economy (SDF & BE) and Kenya Marine Fisheries Research Institute (KMFRI), Naivasha for provision of the data and information.
REFERENCES
| [1] | Harper DM, Morrison EH, Macharia MM, Mavuti KM, Upton C. Lake Naivasha, Kenya: ecology, society and future. Freshw Rev 2011; 4: 89-114. |
| [2] | Gaudet JJ. Seasonal changes in nutrients in a tropical swamp: North Swamp, Lake Naivasha. J Ecol 1979; 67: 953-81. |
| [3] | Verschuren D, Tibby J, Sabbe K, Roberts N. Effects of depth, salinity, and substrate on the invertebrate community of a fluctuating tropical lake. Ecology 2000; 81: 164-82. |
| [4] | Becht R, Odada EO, Higgins S. Lake Naivasha: experience and lessons learned. In: Managing lakes and basins for sustainable use, a report for lake basin managers and stakeholders. Kusatsu: International Lake Environment Committee Foundation (ILEC) 2005; pp. 277-98. |
| [5] | Oyugi D, Mavuti KM, Aloo PA, Ojuok JE, Britton JR. Fish habitat suitability and community structure in the equatorial Lake Naivasha, Kenya. Hydrobiologia 2014; 727: 51-63. |
| [6] | Hickley P, Bailey R, Harper DM, et al. The status and future of the Lake Naivasha fishery, Kenya. Hydrobiologia (Dev Hydrobiol 168) 2002; 488: 181-90. |
| [7] | Hickley P, Muchiri M, Boar RR, et al. Habitat degradation and subsequent fishery collapse in Lakes Naivasha and Baringo, Kenya. Ecohydrol Hydrobiol 2004; 4: 503-17. |
| [8] | Britton JR, Harper DM, Oyugi DO, Grey J. The introduced Micropterus salmoides in an equatorial lake: a paradoxical loser in an invasion meltdown scenario? Biol Invas 2010; 12(10): 3439-48. |
| [9] | Kundu R, Aura CM, Muchiri M, Njiru M, Ojuok JE. Difficulties of fishing at Lake Naivasha, Kenya: is community participation in Management the Solution? Lakes Reserv: Res Manage 2010; 15(1): 15-23. |
| [10] | Kitaka N, Harper DM, Mavuti KM. Phosphorus inputs to Lake Naivasha, Kenya, from its catchment and the trophic state of the lake. Hydrobiologia 2002; 488: 73-80. |
| [11] | Ramsar. The list of Wetlands of International Importance 2014. Available from: http://www.ramsar.org/pdf/sitelist.pdf [Accessed on: 6 July 2016]. |
| [12] | Boar RR. Responses of a fringing Cyperus papyrus L Swamp to changes in water level. Aquat Bot 2006; 84: 85-92. |
| [13] | Aloo PA, Oyugi DO, Morara GN, Owuor MA. Recent changes in fish communities of the equatorial Lake Naivasha, Kenya. Intern J Fish Aqua 2013; 5(4): 45-54. |
| [14] | Ojuok J, Njiru M, Mugo J, Morara G, Wakwabi E, Ngugi C. Increase dominance of common carp, Cyprinus carpio L: the boom or the bane of Lake Naivasha fisheries? Afr J Ecol 2008; 46(3): 445-8. |
| [15] | Muchiri SM, Hickley P. The fishery of Lake Naivasha, Kenya. In: Cowx IG, Ed. Catch effort sampling strategies: their application in freshwater fisheries management. Oxford: Fishing News Books, Blackwell Scientific Publications 1991; pp. 382-92. |
| [16] | Muchiri SM, Hickley P, Harper DM, North E. The potential for enhancing the fishery of Lake Naivasha, Kenya. In: Cowx IG, Ed. Rehabilitation of freshwater fisheries. Oxford: Fishing News Books, Blackwell Scientific Publications 1994; pp. 348-57. |
| [17] | Scott WR, Crossman EJ. Freshwater fishes of Canada. Bull Fish Res Board Can 1973; 184: 1-966. |
| [18] | Parkos J, Santucci VJ Jr, Wahl D. Effects of common carp (Cyprinus carpio) on multiple trophic levels in shallow mesocosms. Can J Fish Aquat Sci 2003; 60: 82-192. |
| [19] | Petr T. Interactions between fish and aquatic macrophytes in inland waters: a review. Italy: FAO Fisheries Technical Paper 2000; (396): 185. Available at: http://trove.nla.gov.au/work/1185996 |
| [20] | Lahmeyer J. Kenya historical demographical data of the administrative division, Available from: http://www.populstat.info/ Africa/kenyap.htm 2002. [Accessed on: 30th May 2016] |
| [21] | Economic Survey. Nairobi, Kenya: Government printer 2009, ISBN: 9966-767-52-5. |
| [22] | Mireri C. Proceedings FWU topics of integrated watershed management. Challenges Facing the Conservation of Lake Naivasha, Kenya GTZ Dar es Salaam 2005; 3: 89-98. |
| [23] | Mutia T, Virani M, Moturi W, Muyela B, Mavura W, Lalah J. Copper, lead and cadmium concentrations in surface water, sediment and fish, Cyprinus Carpio, samples from Lake Naivasha: effect of recent anthropogenic activities. Environ Earth Sci 2012; 67(4): 1121-30. |
| [24] | Njogu PM. Assessment of pollution and prediction of environmental risks of organochlorine pesticide residues on aquatic communities in lake Naivasha, Kenya. Doctoral dissertation, Jomo Kenyatta University of Agriculture and Technology 2014. |
| [25] | Gudka M. Assessment of pesticide concentrations in environmental and biological parameters from two Kenyan Rift Valley Lakes. South Africa: University of Cape Town 2012. |
| [26] | Njiraini GW, Guthiga PM. Are small-scale irrigators water use efficient? Evidence from lake Naivasha basin, Kenya. Environ Manage 2013; 52(5): 1192-201. |
| [27] | Harper DM, Mavuti KM. Lake Naivasha, Kenya: ecohydrology to guide the management of a tropical protected area. Ecohydrol Hydrobiol 2004; 4: 287-305. |
| [28] | Hickley P, Britton J, Macharia S, Muchiri SM, Boar RR. The introduced species fishery of Lake Naivasha, Kenya: ecological impact vs socio-economic benefits. Fish Manag Ecol 2015; 22(4): 326-36. |
| [29] | Nable RO, Banuelos GS, Paull JG. Boron toxicity. Boron in Soils and Plants. Plant Soil 1997; 198: 181-98. |
| [30] | Ballot A, Kotut K, Novelo E, Krienitz L. Changes of phytoplankton communities in Lakes Naivasha and Oloidien, examples of degradation and salinization of lakes in the Kenyan Rift Valley. Hydrobiologia 2009; 632: 359-63. |
| [31] | Njiru J, Morara G, Waithaka E, Mugo J. Fish Kills in Lake Naivasha, Kenya: What was the probable cause? Inter J Fish Aquat Stud 2015; 3(1): 179-84. |
| [32] | Britton JR, Boar R, Grey J, Foster J, Lugonzo J, Harper DM. From introduction to fishery dominance: the initial impacts of the invasive carp Cyprinus carpio in Lake Naivasha, Kenya, 1999-2006. J Fish Biol 2007; 71: 239-57. |
| [33] | Lake NM. Lake Naivasha management plan. Lake Naivasha basin integrated management Plan 2012-2022 2012. Available at: ftp://ftp.itc.nl/pub/naivasha/imarisha/LNB_Mgt_Plan_28March2012.pdf[uploaded 25th Aug 2016]. |
| [34] | Nunan F. Governance and fisheries co-management on Lake Victoria: challenges to the adaptive governance approach. Mast 2010; 9(1): 103-25. |
| [35] | The Water Act. Nairobi, Kenya: Kenya Government Printer No. 8 of 2002. (2002b). |
| [36] | Mageria C, Bosma R, Roem A. Aquaculture development potential in and around Lake Naivasha, Kenya. Netherlands, Wageningen University. 2006. |
| [37] | The Economic Impact of Travel and Tourism 2014. World Travel and Tourism Council. Available at: http://www.wttc.org/%20-/media/ files/reports/economic%20impact%20research/country%20reports/kenya2014.pdf [Accessed on: May 17, 2016]. |
| [38] | Pegram G. Shared Risk and opportunity in water resources: Seeking a sustainable future for Lake Naivasha WWF Report. Pegasys Strategy & Development 2011; p. 34. |
| [39] | Akama JS. Neo-colonialism, dependency and external control of africa's tourism industry. London, UK: Taylor & Francis 2004. |
| [40] | Marwijk Van R, Joosten M. A Small contribution? Small tourism entrepreneurs and sustainable development in Malindi, Netherlands: Wageningen University, 2003. |
| [41] | Ikiara M, Okech C. Impact of tourism on environment in Kenya: status and policy. In: KIPPRA discussion paper number 19. Kenya Institute for Public Policy Research and Analysis. 2002. |
| [42] | Gupta N, Shannon DB, Raghavan B, Danylchuk AJ, Steven JC. Status of recreational fisheries in India: Development, issues, and opportunities. Rev Fish Sci Aquacul 2015; 23(3): 291-301. |
| [43] | Gupta N, Bower S, Cooke SJ, Danylchuk A, Raghavan R. Practices and attitudes of Indian catch-and-release anglers: identifying opportunities for advancing the management of recreational fisheries. J Threat Taxa 2016; 8(4): 8659-65. |
| [44] | The Fisheries Management and Development Act. Nairobi: Government Printer 2016, Keinya Gazette Supplement No. 156 (Act No. 35). |
| [45] | Gupta N, Rajvanshi A, Sathyakumar S, et al. Climate change and Himalaya: Need for targeted education programme for preparedness and formulating adaptive strategies. Cur Sci 2015; 109(7): 1233-4. |